-
 EN
EN

 EN
EN

Service
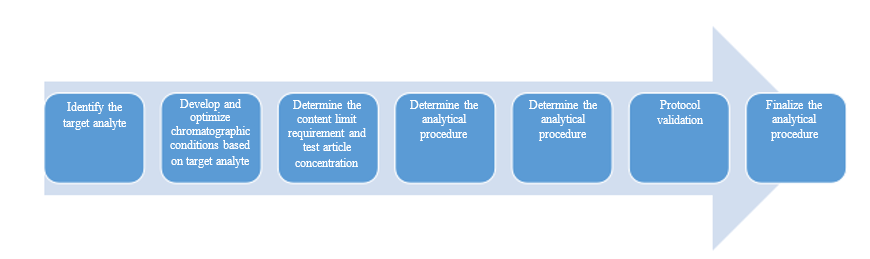
Development and Validation of Analytical Methods
We have a wide range of chromatographic techniques (HPLC, UPLC, GC, IC) and detectors (UV, PDA, ELSD, RID, RF, FID, TCD, MSS) to meet the development needs of analytical methods for different types of compounds and various drug dosage forms and strengths, and can perform method validation of the developed methods according to the requirements of ICH, ChP, USP or EP ( specificity, linearity/range, accuracy, precision, robustness, detection limit, quantification limit, membrane adsorption, solution stability, etc.) to establish reliable and practical analytical methods, mainly applied to the test of essay, related substances, dissolution (profile), and residual solvents for all drug dosage forms. We are particularly good at the development and validation of analytical methods for small strengths (0.025 mg, 0.1 mg and 1 mg).
Development and Validation Process for Routine Analytical Methods: